Advancing Life Science Manufacturing for Our Customers
Life Sciences
- Diabetes care
- Point of care diagnostics cartridge
- Wearable technology
- Vascular access catheter
- Surgical devices & tools
- Medical device battery
- Personal care products

Stäubli Sterimove Integration Partner
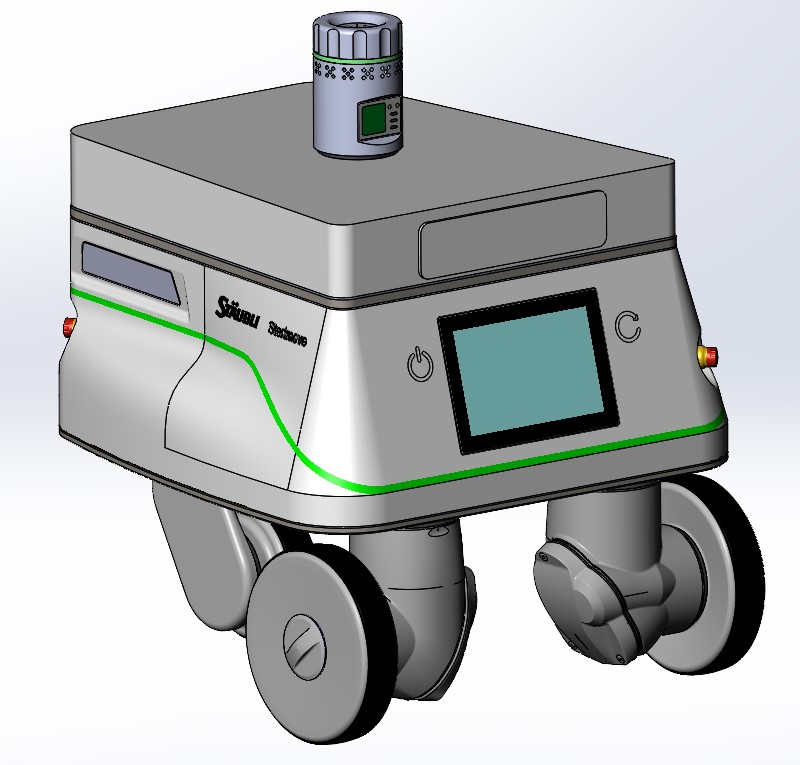
A portable air sampling unit is mounted to the Sterimove to allow for continuous monitoring in cleanroom environments.
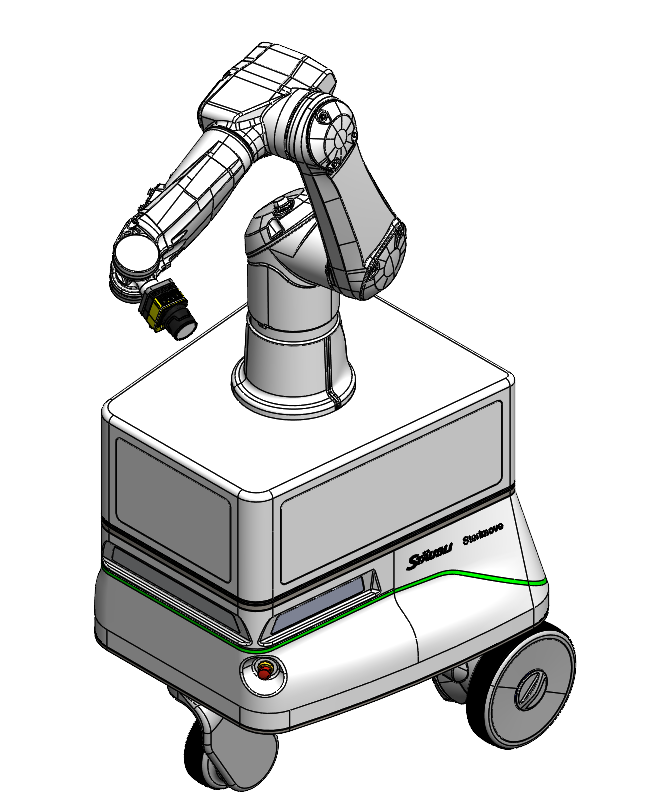
This robot mounted camera allows for an operator or station to request on demand inspection. The AMR is signaled to required inspection area and the robot arm moves to required stand off distance for inspection.
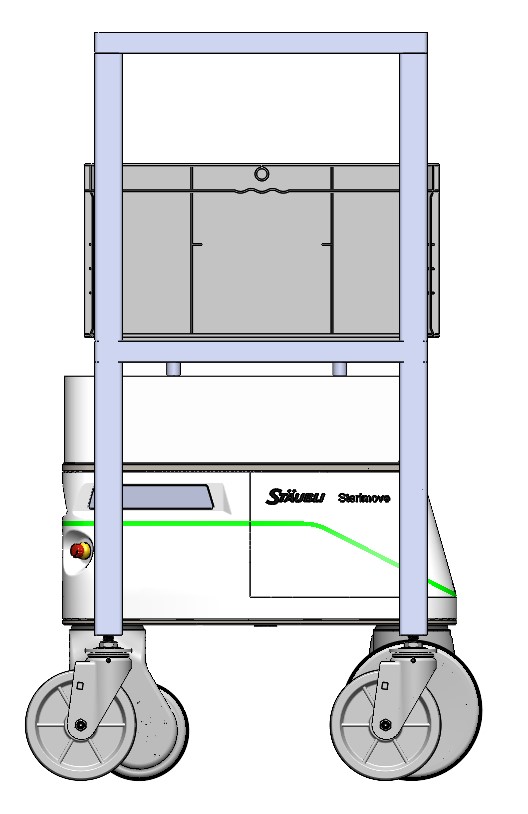
The Sterimove topper has engageable pins to locate and transport racks across the cleanroom. The AMR can transport material based on programmable work instructions
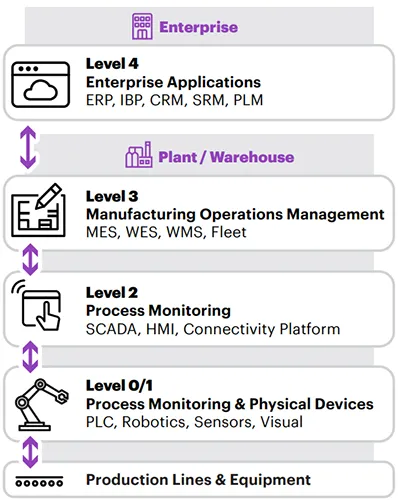
Life Sciences Technology Stack
What is a Technology Stack?
A platform that can be extended with components to support intelligent operations
- coordinate manufacturing and warehouse execution processes
- orchestrate manual and autonomous activities (workers, robotic arms, AMRs, humanoids, etc.)
What are the benefits?
Unlock value from the investment in automation solutions
- optimize how the available resources are used
- orchestrate such resources to efficiently and effectively fulfill the manufacturing and/or warehouse execution plans
Eclipse GAMP Process
- Follows GAMP “V” Model
- Eclipse ISO procedures and Documentation follow guidelines Of GAMP
- Eclipse detailed Functional Spec, Design Spec and VATP facilitate easy creation of IQ and OQ Documents
- Eclipse can provide support in:
- Creation of URS
- Creation of IQ, OQ docs
- Equipment support during IQ, OQ, PQ Validation phase

Our Competitive Advantage
We build state-of-the-art solutions that exceed expectations, support investment plans and strengthen manufacturing strategies.
Continuous blood glucose monitor assembly
- 4 second Takt time
- New product launch CGM Sensor & Applicator
- 10 Lines ($110m)
- Process Development for volume automation
- Initial system Design, Build, Integrate
- Bluetooth Communications and Testing
- GAMP Documentation
- Installations, Service, Spare Parts – Globally
- Validation and Early Production support
- Continuous Improvements
- Locations in US, Mexico, Malaysia – Globally Supported
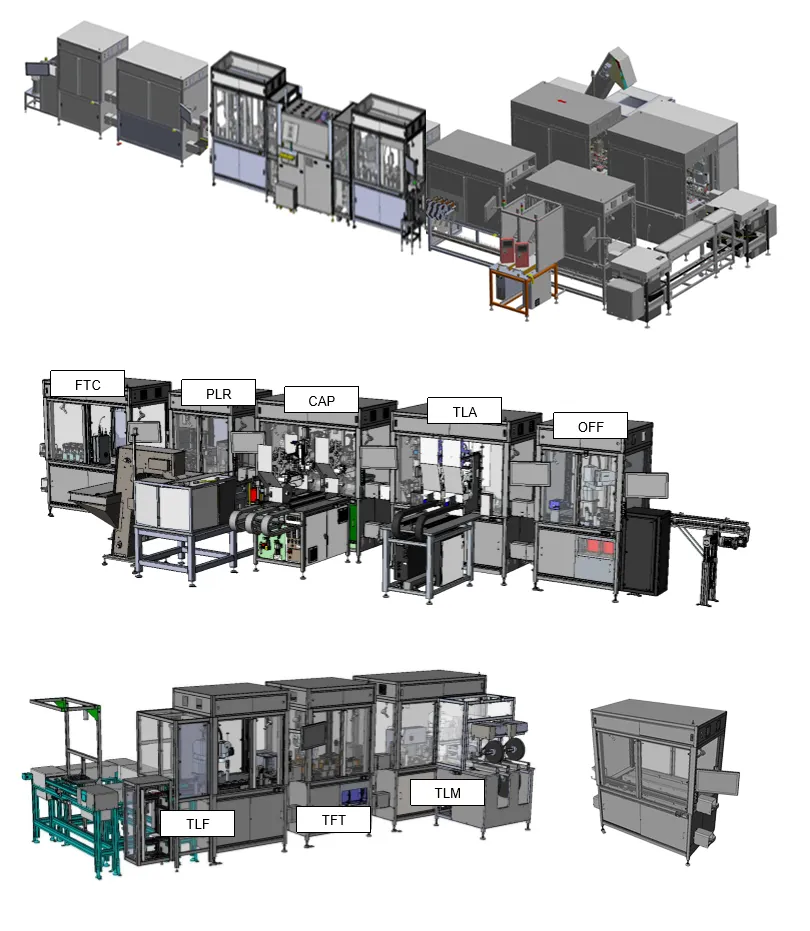
Wearable cardiac assembly
- 5.5 second cycle time
- Semi automated assembly of single use cardiac monitor
- URS Development
- SCADA System-MES interface
- Load of top and bottom plastic housings
- Laser mark and verify
- Battery test, load
- Adhesive application
- Load PCBA
- Device Test
- Integration to Pouching machine
- GAMP documentation

High volume cartridge filling application
- 20,000 parts per hour per machine
- Pre-Automation Proof of Concept involvement
- FDA 21 CFR 11 Compliant Systems with Audit trails.
- Developed new filling process to decrease dispense time from 15s to 1.6s
- Deterministic Data Management implementation
- End to End Traceability of all incoming materials to the final Assembly
- High accuracy fluid dispense 0.78g ± 2%
- Repeat build of 20 full Systems ($70m)
- Facilitating Customer in 3rd Party Equipment Specification and final Integration and Test.
- Management of over 40 different Recipes
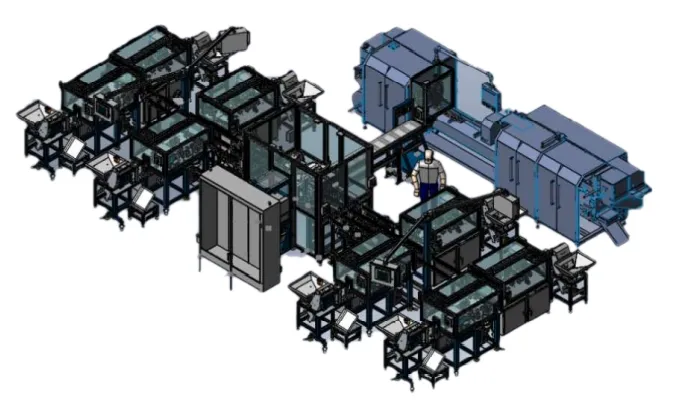
Needle overmolding
- 6 second cycle time
- Robot 1 – 4 Cavity Mold Unload
- Rotate and Pitch Assembly
- Robot 2 – Load to Tray
- Tray Handling Conveyor
- Inspection of tip for burrs
- Inspection of needle in mold position
- Verify mold position on dial
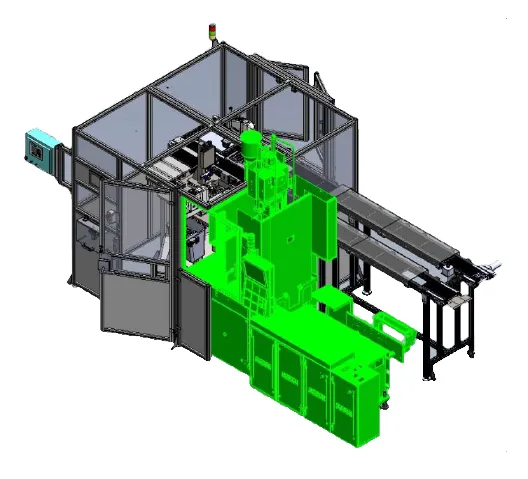
Introducer sheath assembly
- 6 second cycle time
- ISO Class 8 & Design for wipe down
- 2 Parts to line: Sheath Sub Assembly, Final Assembly
- 3 Product Lengths
- Precision Indexing Chassis
- Tube cutting & loading
- Installation of 3 way stop-cock
- Install Sheath Sub Assembly
- Vision Inspection, Tube Insert Depth, Apply Glue
- Vapor set epoxy

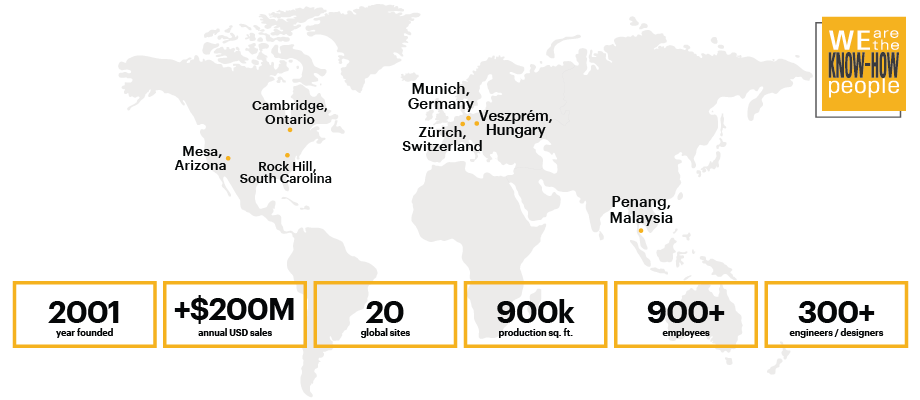
Who We Are
Start the Conversation

Grant Townsend
Manager, N.A. Industry Sales – Life Sciences
Email: Grant Townsend
Call: 803.415.5626
