How Eclipse Automation Can Take Your COVID-19 Diagnostic Test Production to the Next Level
2020 was a year like no other in recent memory. The global spread of the novel coronavirus COVID-19 and its ramifications permeated every aspect of society. Global health care resources were stretched thin like never before. The demand for aid was heard loud and clear – and many organizations answered the call. Companies around the world pivoted and accelerated manufacturing of everything from hand sanitizer to urgently-needed personal protective equipment and life-saving medical ventilators.
In these uncertain times, the medical device manufacturing sector has been put under immense pressure, forced into highly compressed development timelines. The results of these urgent timelines have been acutely felt by manufacturers of COVID-19 point-of-care (PoC) diagnostic testing devices.
As the pandemic raged on, the need for accurate testing quickly became a priority, and has been vital in Canada’s overall response strategy. The combination of nation-wide testing and a host of public health measures – including mask-wearing and social distancing – has formed the backbone of our country’s COVID-19 response plan.
It is precisely in these challenging times that medical device manufacturers and organizations have the most need for a reliable, established, and innovative automation partner. Enter Eclipse Automation.
An Urgent Need – A Trusted Partner
In 2020, the race for Life Sciences companies around the world to rapidly produce COVID-19 testing solutions pushed biomedical manufacturers to their limits. In response to the pandemic, the pace of product development and launch for point-of-care diagnostic testing related to the detection of COVID-19 required companies to deploy products at an extraordinary rate.
Today, Life Sciences organizations need a full solution automation provider who can be a true partner – one that not only builds machines, but aids in the development of a holistic manufacturing plan. A partner that can enable them to take their production to the next level, and help protect the people and communities which they serve. A partner like Eclipse Automation.
Three Challenges – Our Solutions
Here at Eclipse, we are applying our trusted expertise and more than two decades of design and manufacturing experience to aid in the COVID-19 crisis and help build a better Next Normal. We are partnering with and assisting several customers through manufacturing challenges with the launch of their COVID-19 point-of-care diagnostic testing products.
Three common challenges that these manufacturers often face are:
- Navigating the logistics of transitioning the manufacturing process from manual assembly
- Lack of strategy for ramping up manufacturing volume
- Lack of a plan to reach FDA Validation
For each of these, we provide solutions that are tailored to manufacturers’ specific and unique needs.
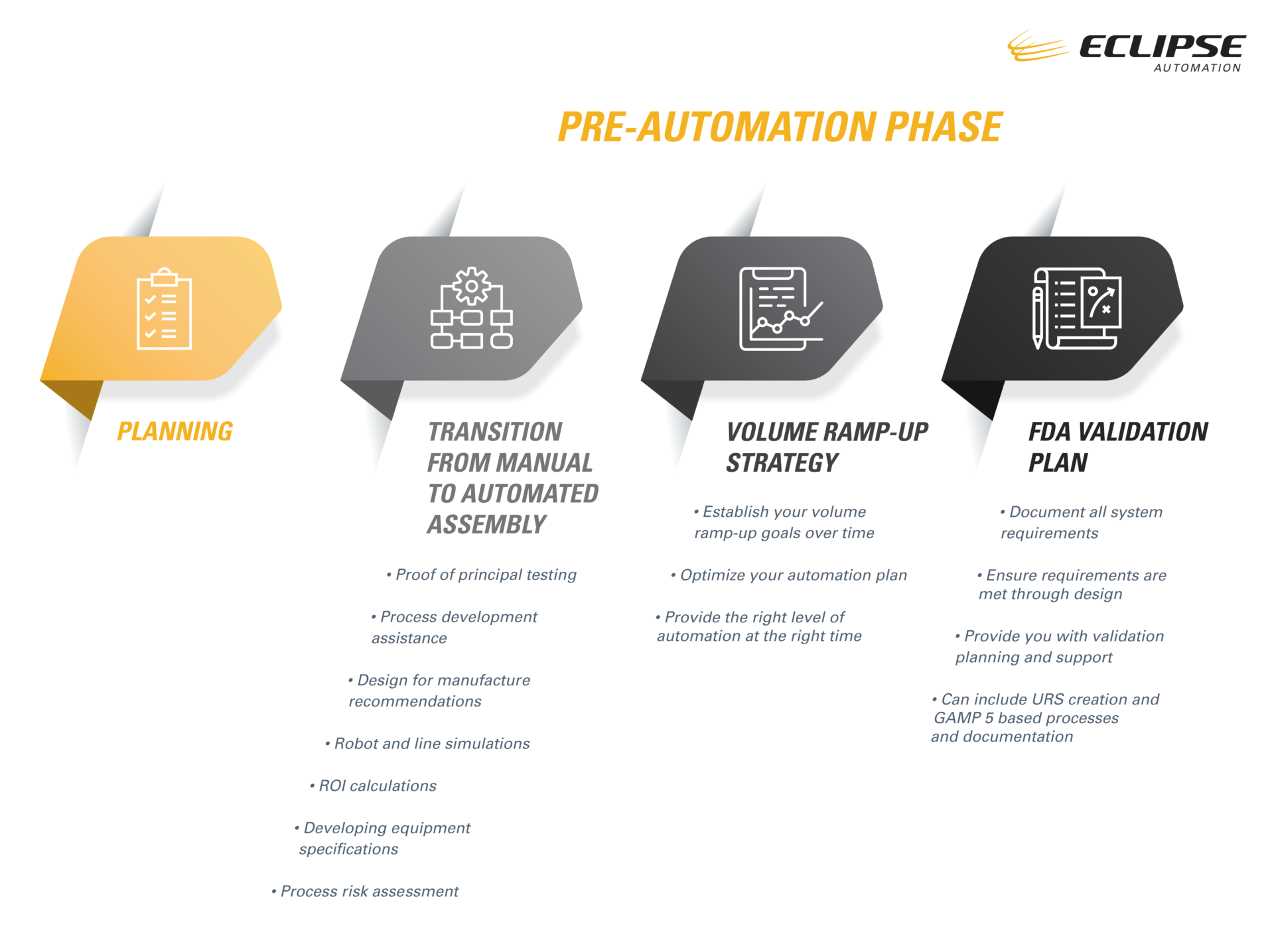
Transitioning from Manual to Automated Assembly
As Life Sciences organizations work tirelessly to develop much-needed medical equipment at an increasingly fast pace, Eclipse Automation provides vital assistance in transitioning their manufacturing processes from manual assembly – typically used in prototype and early trials – to semi-automated and fully automated processes.
The transition from manual to semi or fully automated assembly carries unique risks and challenges. This is especially true when manufacturers are under pressure to develop and deploy their equipment in a compressed time frame. Difficulties in automatic assembly of intricate parts often require the kind of articulation and optical guidance that only a trusted, experienced automation partner can provide. Here at Eclipse, we are poised and ready to assist your organization in developing automated processes for your COVID-19 point-of-care diagnostic testing solutions.
To enable your project to reach its full potential, we offer thorough pre-automation services, including early proof of principal testing. As design and building of equipment takes place, the longer a manufacturer waits to test out new systems, the higher the chances that their roll-out schedule may be negatively affected. This could lead to delays in deployment – a critical misstep for medical device manufacturers in the race to produce urgently-needed COVID-19 diagnostic testing solutions. Our early proof of principal testing helps to mitigate process risks at minimal cost to your organization.
As Director of Business Development Chris Knorr explains, “Rather than waiting until the integration phase for testing, spending the necessary time in the early stages of the project – prior to design – characterizing new processes and proving automated methods will save exponential cost and schedule.”
Besides proof of principal, our other pre-automation services include:
- Process development assistance
- Design for manufacture recommendations
- Robot and line simulations
- ROI calculations
- Developing equipment specifications
- Process risk assessment
Developing a Volume Ramp-Up Strategy
Since the onset of the pandemic, companies producing COVID-19 diagnostic testing solutions have been racing to get to market at a remarkable rate. The balance between quick deployment and risk mitigation is vital. Transitioning from manual to high volume automation carries its own risks – and the higher the volume of automation, the more risk is present.
Here at Eclipse Automation, we understand the challenges in striking that balance – that’s why we provide assistance in the creation and development of a manufacturing volume ramp-up plan that is tailored to your project.
To create the most effective strategy, we first seek to understand our customers’ volume ramp-up goals over time. We then optimize their automation plan by balancing their time to market goals, often through the use of semi-automated equipment. In using this approach, we can quickly provide our clients with the right equipment, which enables them to get a portion of volume to market within accelerated timelines and at a fairly low risk.
We then work with the customer to understand their product forecasts. We collaborate on developing a plan for ramp-up and determining when to introduce full automation into the process. In establishing this timeline, we work hand-in-hand with customers to strike a balance between deploying the best approach while taking into account their long-term manufacturing needs. We consistently strive to get our clients the best possible return on investment by providing the right level of automation at the right time, with the end goal of optimizing their manufacturing through a higher degree of automation in order to reduce long-term labour operating costs.
Establishing an FDA Validation Plan
Now more than ever, FDA validation is a critical path process step in bringing new medical devices to market. Newer, smaller, or short-staffed organizations may encounter challenges with bringing their product to market and getting it validated. That’s why it is so critical to have a partner who can assist you in the creation of a clear, actionable validation plan. We provide added value by documenting all system requirements and ensuring these requirements are met through design. We can assist your organization in providing validation planning and support that’s tailored to your needs through URS creation as well as GAMP 5 based process and documentation.
“Equipment validation planning is something that often gets little attention in the early stages of bringing a new product to market,” says Knorr. “However, through early documentation of the critical to quality parameters, equipment requirements, how those requirements will be met through design, and how they will be tested, we ensure that the equipment will meet your manufacturing needs while reducing the risk of critical path issues during the late stages of testing and validation.”
We work closely with your organization, helping shorten the validation phase by providing the right assistance at each touchpoint, enabling you to achieve that critical goal – getting your COVID-19 diagnostic testing solutions to market faster.
Vertical Integration: The Eclipse Advantage
With our total vertical integration model, we have the ability to deliver exceptional results through our in-house processes from conception to installation, providing superior quality control, simplified logistics, seamless communication, and a scalable scope for your project.
Our in-house departments of design, fabrication, machining and assembly enable us to optimize all aspects of your project and provide you with accurate quoted lead times, prioritized assemblies and optimized project schedules, as well as superior quality control for each solution provided. We ensure the highest quality standards for each customer and guarantee efficient and innovative solutions every step of the way.
COVID-19 Point-of-Care Diagnostics: Our Approach
Point-of-care diagnostic testing detects COVID-19 antigens or nucleic acids and provides results on-site in as little as fifteen minutes to an hour. This eliminates the longer wait times needed with traditional lab testing methods such as the widely-used lab-based PCR (polymerase chain reaction) test. PCR tests must be processed in a lab, which can take at least 24 hours to provide results.
At Eclipse, our superior processes in pre-automation, custom automation and post-automation services guarantee the utmost quality for your COVID-19 point-of-care diagnostic testing solutions.
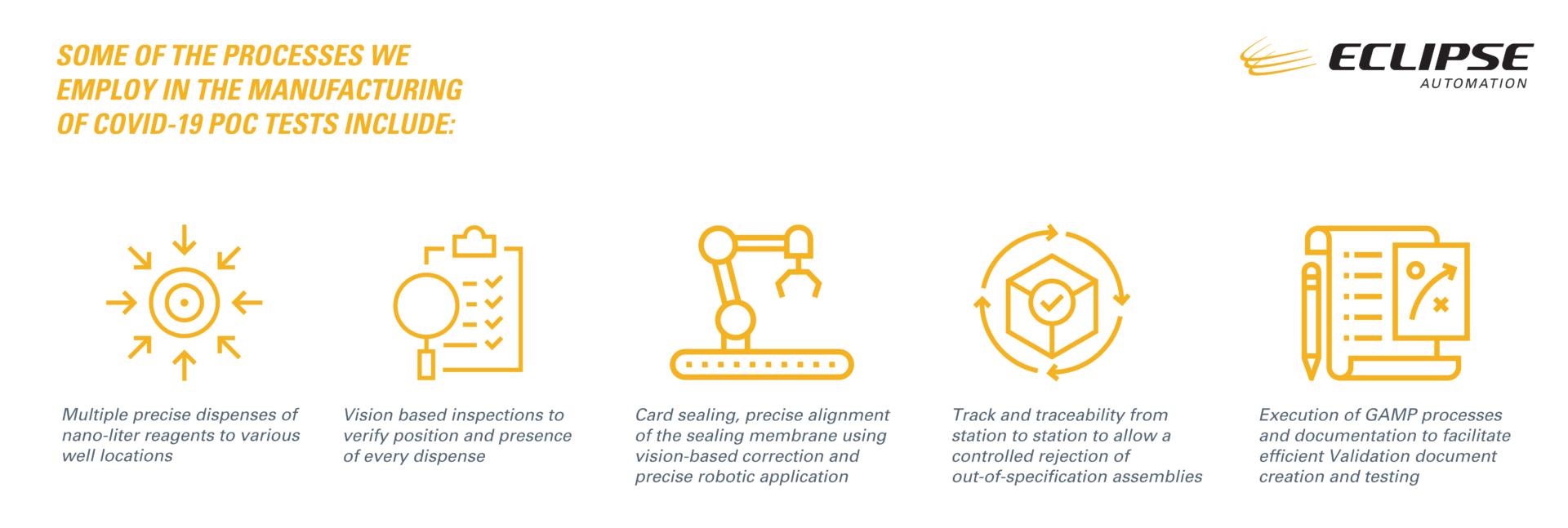
Some of the processes we employ in the manufacturing of COVID-19 PoC tests include:
- Multiple precise dispenses of nano-liter reagents to various well locations.
- Vision based inspections to verify position and presence of every dispense.
- Card sealing, precise alignment of the sealing membrane using vision-based correction and precise robotic application.
- Track and traceability from station to station to allow a controlled rejection of out-of-specification assemblies.
- Execution of GAMP processes and documentation to facilitate efficient Validation document creation and testing.
Our Expertise – Your Product
Your organization deserves an automation partner that you can count on – a partner that will face the challenges of today and tomorrow head-on.
At Eclipse Automation, we have assisted multiple medical device manufacturing customers through their new COVID-19 point-of-care diagnostic test product launch manufacturing challenges. We have the proven experience and depth of resources to be the ideal automation solution partner to bring your products to market.
Our innovation process combines two decades of design and manufacturing expertise to help you connect the dots and get your product to market sooner. From product concepts and building, to testing and launching, our experienced global team of engineers, designers, developers and project managers use their technical skills to bring your project to life with maximized yield and cycle times while delivering cost efficiencies and superior user experience.
Our team of over 750 experts across manufacturing locations in Canada, the United States and Europe have the skills to deliver systems from single semi-automated cells to large multiline programs. With 20 years of complex custom automation experience across a wide range of industries, we are poised and ready to help your organization reach its highest potential and optimize your COVID-19 point-of-contact diagnostics test manufacturing plan. We will collaborate with your organization to ensure that your end product reaches its ultimate goal – assisting in the global fight against the COVID-19 pandemic.
To learn more or request a quote, please visit:
